Interested in distribution opportunities with Shira? Please complete a Contact form and review our territory map.
Why Partner With Shira
- Diverse product lines that can satisfy distinct channel needs
- Pricing strategy that permits attractive margins for resellers and value for consumers
- 20-year track record of innovative product development
- Reputation for excellence among spa-industry professionals
- Marketing tools, support, and sampling to help assure success
- Continuing education on products and treatment protocols
Tell us about yourself by completing the Contact form.
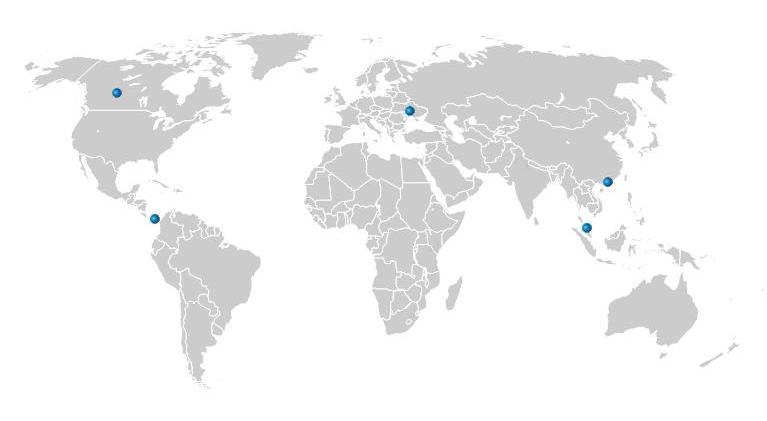
Review locations where Shira already has distributor relationships
United States
Shira Esthetics - Corp. Hdqtrs.
65 S. 21st. St.
Kenilworth, NJ 07033
1-800-957-4472
United States
California Skin Care Supply
340D El Pueblo Rd.
Scotts Valley, CA 95066
1-800-500-1886
Canada
Fernanda's Beauty Products
5080 Timberlea Blvd.
Mississauga, Ontario L4W4M2
800-862-1447
Ukraine
Shira Esthetics Ukraine LLC
T. Yablonskoy 22 str.
Kiev, Ukraine 03058
+38 044 228 86 56
 Advanced Skin Care for Exceptional Results
Advanced Skin Care for Exceptional Results